
“The FDA and other regulatory bodies are tightening their scrutiny of traceability and chain of custody for clinical research and trials,” according to Brian O’Dwyer, CEO of Q2 Solutions.
“There is no industry standard regarding the exchange of chain of custody information. DLT is a great technology for ensuring trust in the data.”
The need
In an environment, with increasingly specialised opportunities, a complex web of local and international regulatory hoops to navigate and unrelenting shareholder and market scrutiny, it is paramount for drug companies to bring products to market quickly and reliably. But to do this, trials are mandatory.
From a clinical trials perspective, the quest for efficiency and specialisation has driven pharmaceutical companies to seek the expertise of Contract Research Organisations (CROs) which then carry the responsibility of:
- meeting timelines
- ensuring quality assurance
- satisfying data control requirements.
CROs in turn look to sample laboratories, logistics partners and specialised healthcare professionals to collect, store manage samples and data. As CROs and the drug companies expand beyond the developed world in order to generate diverse and cost-effective networks of trial sites and sources of patients, the difficulty of achieving milestones while preserving acceptable data quality magnifies. This is what the Q2 PoC, conducted with HSBlox on Azure, investigated.
Q2, the PoC and clinical trial
During clinical trials Q2 collects human biological samples at clinical investigator sites over an extended period. Flawless administration of the chain of custody is critical to sample management because correct handling impacts both the course and results of clinical trials. Conversely, defective sample management can involve missing or contaminated samples, incomplete data, delays in reporting results and substandard logistics. Each of these leads to higher costs, lack of transparency as well as other problems.
The participants designed the PoC to investigate if these pitfalls are avoidable with the use of technology like DLT. The PoC, therefore, simulated:
- collection
- transportation
- test result generation
- storage of the sample.
For the POC, a subset of stakeholders represented DLT network participating nodes:
- a transportation courier
- CRO operations
- CRO Laboratory
- a storage facility
- a notary.
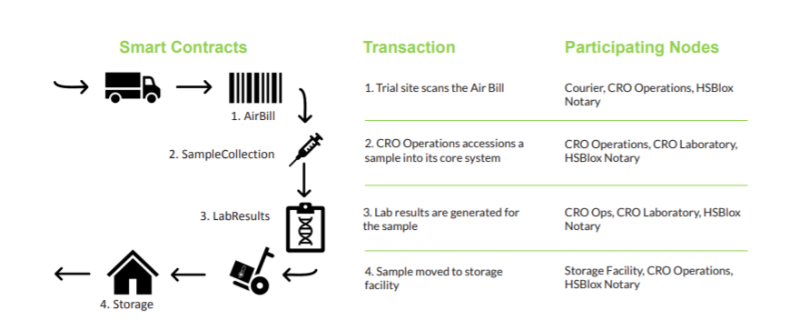
The PoC demonstrated that a blockchain tracking solution can streamline the collection and sharing of information. Multiple clinical trial stakeholders – each with differing systems and data repositories – represent an intelligent distributed network for the gathering and dissemination of clinical and operational data relevant to the trial and its outcome.
The smart contract dimension
HSBlox developed the sample tracking (POC) partnership with a CRO. Using HSBlox’s smart contract solutions added automation, transparency as well as tracking of permissioned data disclosure to the clinical research ecosystem. For the POC, HSBlox’s Digital Sample Manager solution used R3’s Corda blockchain platform deployed in Microsoft Azure.

“This project, which requires highly accurate tracking through a complex chain which might be made up of many different parties, is ideally suited to blockchain. The decentralized nature of blockchain ensures transparency and trust, while Corda’s enhanced privacy and security technology can ensure that sensitive data is handled with the appropriate care,” said David E. Rutter, CEO of R3.
“This innovative application introduces much needed additional controls and clarity to vital scientific research. We look forward to supporting the continued development of this application through the Corda ecosystem.”
Enterprise Times: What does this mean
The PoC concluded that this initiative demonstrated smart contracts can:
- improve the efficiency and transparency of data management with respect to sample management
- eliminate the need to reconcile between entities
- expedite trial audit processes.
- support the transparency and traceability necessary for the validity of clinical trials.
Besides the specific conclusions relevant to clinical trials, what this Q2 solutions/HSBlox PoC also demonstrated is that blockchain solutions work when there are many diverse stakeholders dependent on sequencing, custody and transparency. The same applies in supply chains, for example. As a design principle, this could well emerge as ‘tool’ to assess if a blockchain project has validity: without those many participants sharing in a mutual goal (in this case clinical trial results), a project might not be worthwhile.